How To Calculate Core Electrons
Chapter 4: Periodic Properties of the Elements
[LibreClone] 4.ii Electron shielding and constructive nuclear charge
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/10%3A_Multi-electron_Atoms/Multi-Electron_Atoms/Penetration_and_Shielding
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/08%3A_Periodic_Properties_of_the_Elements/8.06%3A_Periodic_Trends_in_the_Size_of_Atoms_and_Effective_Nuclear_Charge
Learning Objectives
- To sympathise the basics of electron shielding and penetration
Penetration and shielding are two underlying principles in determining the concrete and chemical properties of elements. Nosotros can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends.
Introduction
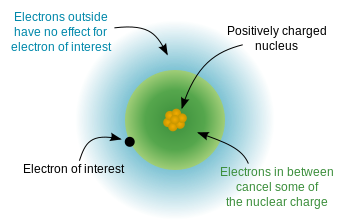
Electrons are negatively charged and are pulled pretty close to each other by their allure to the positive charge of a nucleus. The electrons are attracted to the nucleus at the same fourth dimension every bit electrons repel each other. The rest between bonny and repulsive forces results in shielding. The orbital (n) and subshell (m l) ascertain how close an electron can approach the nucleus. The ability of an electron to get close to the nucleus is penetration.
Coulomb's Law tin can exist used to draw the attraction and repulsion betwixt atomic particles. The force that an electron feels is dependent on the altitude from the nearest charge (i.east., an electron, normally with bigger atoms and on the outer shells) and the amount of charge. More distance between the charges will result in less force, and more charge will take more forcefulness of allure or repulsion. In the simplest case, every electron in an atom would feel the same amount of "pull" from the nucleus. For example, in [latex]\ce{Li}[/latex], all three electrons might "experience" the +3 charge from the nucleus. Notwithstanding, this is non the case when observing diminutive behavior. When considering the core electrons (or the electrons closest to the nucleus), the nuclear charge "felt" by the electrons ( Effective Nuclear Accuse (Z eff)) is close to the actual nuclear charge. Equally you proceed from the core electrons to the outer valence electrons, Z eff falls significantly. This is because of shielding, or simply the electrons closest to the nucleus decrease the corporeality of nuclear charge affecting the outer electrons. Shielding is acquired by the combination of partial neutralization of nuclear charge past core electrons, and past electron-electron repulsion.
The corporeality of charge felt by an electron depends on its distance from the nucleus. The closer an electron comes to the nucleus, or the more it penetrates, the stronger its allure to the nucleus. Core electrons penetrate more and feel more of the nucleus than the other electrons.
$$East=1/(4πϵ_o )⋅(q_1 q_2)/r^2$$
with
- East is the potential energy
- q refers to the charged particles
- r is the separation distance of the particle
- [latex]\epsilon[/latex]o is a constant and is equal to eight.95 × 10-12 C2/Jm
Shielding
An cantlet (assuming its atomic number is greater than 2) has core electrons that are extremely attracted to the nucleus in the middle of the atom. Even so the number of protons in the nucleus are never equal to the number of cadre electrons (relatively) adjacent to the nucleus. The number of protons increase past ane across the periodic table, but the number of core electrons change by periods. The get-go menstruation has no cadre electrons, the second has 2, the tertiary has ten, and etc. This number is non equal to the number of protons. So that means that the core electrons feel a stronger pull towards the nucleus than any other electron within the system. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction.
Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has "less grip" on the outer electrons and are shielded from them. Electrons that accept greater penetration can get closer to the nucleus and finer block out the charge from electrons that have less proximity. For case, Z eff is calculated past subtracting the magnitude of shielding from the total nuclear accuse. The value of Z eff volition provide data on how much of a accuse an electron actually experiences.
Considering the lodge of electron penetration from greatest to least is south, p, d, f; the social club of the amount of shielding washed is also in the society s, p, d, f.
Since the 2s electron has more than density well-nigh the nucleus of an atom than a 2p electron, it is said to shield the twop electron from the full constructive accuse of the nucleus. Therefore the 2p electron feels a lesser outcome of the positively charged nucleus of the atom due to the shielding power of the electrons closer to the nucleus than itself, (i.east. 2s electron).
These electrons that are shielded from the full charge of the nucleus are said to experience an effective nuclear charge (Zeff )of the nucleus, which is some caste less than the full nuclear charge an electron would feel in a hydrogen atom or hydrogenlike ions. The effective nuclear accuse of an cantlet is given by the equation:
Z eff = Z [latex]-[/latex] S
where
- Z is the atomic number (number of protons in nucleus) and
- S is the shielding constant
We can see from this equation that the constructive nuclear charge of an atom increases as the number of protons in an atom increases. Therefore as we become from left to right on the periodic table the effective nuclear charge of an atom increases in strength and holds the outer electrons closer and tighter to the nucleus. As we will discuss later on in the chapter, this phenomenon can explain the decrease in atomic radii we see as nosotros go across the periodic tabular array equally electrons are held closer to the nucleus due to increase in number of protons and increment in effective nuclear accuse.
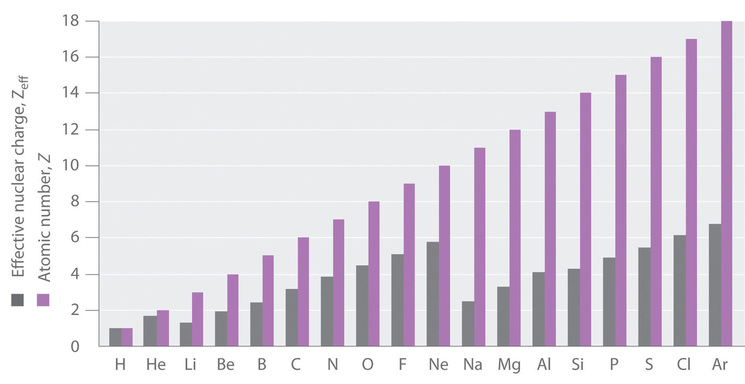
Electron Penetration
The approximation in Equation 1 is a good first gild clarification of electron shielding, simply the actual Zeff experienced by an electron in a given orbital depends not only on the spatial distribution of the electron in that orbital but too on the distribution of all the other electrons nowadays. This leads to large differences in Zeff for dissimilar elements, as shown in Figure 4.2.2 for the elements of the beginning three rows of the periodic tabular array.
Penetration describes the proximity to which an electron can arroyo to the nucleus. In a multi-electron system, electron penetration is defined by an electron'due south relative electron density (probability density) near the nucleus of an cantlet (Figure 4.2.iii). Electrons in dissimilar orbitals take different electron densities around the nucleus. In other words, penetration depends on the crush (n) and subshell (l). Electrons that have greater penetration can get closer to the nucleus and effectively block out the charge from electrons that have less proximity.
For example, a idue south electron (Figure iv.2.3; purple curve) has greater electron density almost the nucleus than a 2p electron (Figure four.2.three; ruddy bend) and has a greater penetration. This related to the shielding constants since the anes electrons are closer to the nucleus than a iip electron, hence the 1s screens a 2p electron virtually perfectly (S = one. Still, the 2s electron has a lower shielding constant (Due south < ane because information technology can penetrate shut to the nucleus in the pocket-size area of electron density within the beginning spherical node (Figure four.ii.3; light-green bend). In this style the 2s electron tin can "avert" some of the shielding effect of the inner 1s electron.
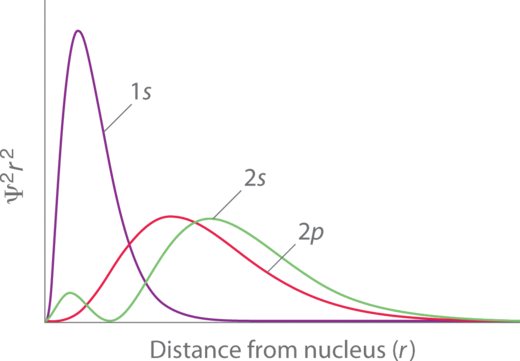
For the same shell value (n) the penetrating power of an electron follows this trend in subshells (Figure 4.two.three):
southward > p > d [latex]\approx[/latex] f.
for dissimilar values of crush (n) and subshell (50), penetrating power of an electron follows this trend:
anes > twodue south > 2p > 3southward > 3p > foursouth > 3d > fourp > 5south > 4d > fivep > 6s > 4f . . .
| Table four.2.ane: Effective Nuclear Charges for Selected Atoms | |||
|---|---|---|---|
| Cantlet | Sublevel | Z | Zeff |
| [latex]\ce{H}[/latex] | 1s | i | 1 |
| [latex]\ce{He}[/latex] | anes | 2 | i.69 |
| [latex]\ce{Li}[/latex] | 1s, 2s | 3 | 2.69, 1.28 |
| [latex]\ce{Be}[/latex] | 1due south, twosouth | 4 | iii.68, 1.91 |
| [latex]\ce{B}[/latex] | 1s, 2s, iip | 5 | 4.68, 2.58, 2.42 |
| [latex]\ce{F}[/latex] | 1s, 2s, 2p | nine | 8.65, v.thirteen, 5.10 |
| [latex]\ce{Na}[/latex] | 1s, 2s, twop, 3s | xi | ten.63, half-dozen.57, 6.eighty, 2.51 |
Data from E. Clementi and D. L. Raimondi; The Journal of Chemic Physics 38, 2686 (1963).
Considering of the effects of shielding and the different radial distributions of orbitals with the same value of north simply unlike values of l, the unlike subshells are not degenerate in a multielectron cantlet. For a given value of n, the ns orbital is always lower in energy than the np orbitals, which are lower in energy than the nd orbitals, and and then forth. As a consequence, some subshells with higher primary quantum numbers are actually lower in energy than subshells with a lower value of due north; for example, the foursouth orbital is lower in energy than the 3d orbitals for most atoms.
Key Concepts and Summary
The calculation of orbital energies in atoms or ions with more than ane electron (multielectron atoms or ions) is complicated by repulsive interactions between the electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the utilise of hydrogen-like orbitals and an effective nuclear charge (Z eff) to describe electron distributions in more complex atoms or ions. The caste to which orbitals with different values of l and the same value of n overlap or penetrate filled inner shells results in slightly dissimilar energies for different subshells in the aforementioned principal shell in most atoms.
Attempt Information technology
- Which orbital is more than constructive in shielding? 1southward or 2p ?
- True/Fake: The greater the penetration of an orbital, the greater the shielding adequacy of that orbital.
- Which of these accept the smallest electron analogousness? [latex]\ce{B}[/latex], [latex]\ce{C}[/latex], [latex]\ce{N}[/latex], [latex]\ce{O}[/latex], or [latex]\ce{F}[/latex].
- Which cantlet has a stronger effective nuclear charge and why? (assuming [latex]\ce{S}[/latex] is the same in both cases) [latex]\ce{Li}[/latex], or [latex]\ce{N}[/latex]
- Why does the Hydrogen electron experiences the total charge of the nucleus without any shielding?
- Which atom has a smaller radii? [latex]\ce{Be}[/latex] or [latex]\ce{F}[/latex]?
- Which electron has higher energy level? 2s or 2p ? and why?
- Why do the orbitals of a hydrogen cantlet increment free energy equally follows: 1s < twos = twop < threesouth = 3p = 3d < 4s = 4p = 4d < . . . .
- Which electrons shields improve in an atom? 2s or 2p ? 3p or threed?
- Why can we relate classical physics to breakthrough mechanics when it comes to subatomic activity?
- What is penetration?
Bear witness Selected Solutions
- anes
- T
- nitrogen atom has a stronger effective nuclear accuse than lithium due to its greater number of protons in the nucleus holding the electrons tighter.
- Hydrogen cantlet has just 1 electron total, therefore at that place are no other, lower energy (more penetrating), electrons available to assist shield this electron from the nucleus.
- Fluorine has a smaller radii than Beryllium due to its greater number of protons providing a greater constructive nuclear accuse on the outer electrons and therefore pulling them in tighter and providing a smaller atomic radii.
- 2p has college free energy level because the negatively charged electron experiences less of an effective nuclear charge than the twodue south electron.
- because a Hydrogen atom has only one electron, that experiences no shielding from other electrons and therefore its free energy level just depends on its distance away from the nucleus, which is dependent on it value of (north).
- iis shields the atom better than 2p because the due south orbitals is much closer and surrounds the nucleus more than the p orbitals, which extend farther out. 3p shields better than threed considering p orbitals are closer to the nucleus than the 3d orbitals.
- Classical physics and breakthrough mechanics both tin deal with subatomic activity such as electron interactions, orbital location, size, and shape, and distances to find forces of attractions.
- Penetration is how well the outer electrons are shielded from the nucleus by the core electrons. The outer electrons therefore feel less of an attraction to the nucleus.
Glossary
- Atomic Radius: The atomic radius decreases from left to right, and increases from tiptop to bottom.
- Constructive Nuclear Charge (Z eff): The effective nuclear charge increases from left to right and increases from top to bottom on the periodic table.
- Electron penetration: The ability of an electron to get close to the nucleus, which is defined past the orbital ( northward ) and subshell ( m l ) on how close an electron can approach the nucleus
Contributors and Attributions: The LibreTexts libraries are Powered by MindTouch®and are supported by the Department of Educational activity Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We too admit previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Unless otherwise noted, LibreTexts content is licensed by CC By-NC-SA three.0. Legal. Have questions or comments? For more data contact us at info@libretexts.org or check out our status page at https://status.libretexts.org.
References
- Petrucci, Ralph H., William Due south. Harwood, F. Geoffrey Herring, and Jeffry D. Madura. General Chemistry: Principles and Modern Applications, Ninth Edition. Pearson Education Inc. Upper Saddle River, New Jersey: 2007.
- Raymond Chang. Physical Chemistry for Biological Sciences. Sausalito, California: University Science Books, 2005
- R. Due south. Mulliken, Electronic Structures of Molecules and Valence. II General Considerations, Physical Review, vol. 41, pp. 49-71 (1932)
- Anastopoulos, Charis (2008). Particle Or Wave: The Development of the Concept of Matter in Modern Physics. Princeton University Press. pp. 236–237. ISBN0691135126. http://books.google.com/?id=rDEvQZhpltEC&pg=PA236.
Contributors and Attributions
- Sidra Ayub (UCD), Alan Chu (UCD)
Licenses and Attributions (Click to expand)
CC licensed content, Shared previously
- North/A. Provided by: North/A. Located at: N/A. License: CC By: Attribution. License Terms: N/A
How To Calculate Core Electrons,
Source: https://pressbooks.online.ucf.edu/chemistryfundamentals/chapter/penetration-shielding-chemistry-libretexts/
Posted by: daviskniout.blogspot.com


0 Response to "How To Calculate Core Electrons"
Post a Comment